|
|
|
| Product Name |
Perfluoro(2-methyl-3-pentanone) |
| Model No |
SIL308F |
| Cas No. |
756-13-8 |
| Assay |
99.7% |
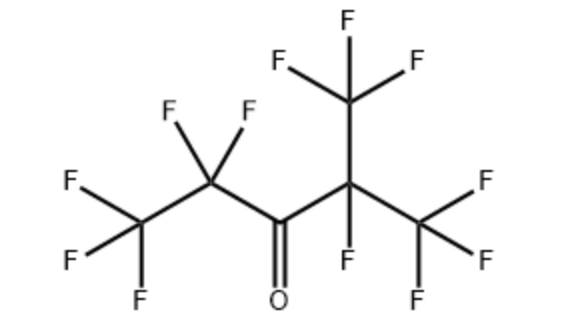
| Molecular Structural |
 |
| Details: |
Product Name:Perfluoro(2-methyl-3-pentanone)
CAS:756-13-8
MF:C6F12O
MW:316.04
Melting point’╝Ü-108°C
Boiling point’╝Ü49°C
density’╝Ü1,6 g/cm3
vapor pressure’╝Ü31.6-40.4kPa at 20-25Ōäā
solubility’╝ÜChloroform (Soluble), Methanol (Sparingly)
form’╝Üclear liquid
Specific Gravity’╝Ü1.6
Water Solubility’╝Ü 24-332.6mg/L at 25Ōäā
Uses’╝ÜPerfluoro(2-methyl-3-pentanone) has outstanding flame retardant effect to prevent burning and is used in the protection of molten magnesium and fire extinguishing agents with no impact on the environment.
158 g of perfluoro-2,3-epoxy-2-methyl pentane, 2.9 g of potassium fluoride, 6 g of sulfolane and 100 mL of diglyme are added into a 500 mL three-neck flask equipped with a reflux condensing tube and a mechanical stirring device, respectively. The temperature is raised to be 30 °C after stir starts. After 3 hours of reaction, the reaction product of Perfluoro(2-methyl-3-pentanone) is analyzed by gas chromatography.
Perfluoro(2-methyl-3-pentanone) can be used as a wet cleaning agent to clean steam reactors and their components, as well as to clean unwanted deposits accumulated in gas phase reactors and etch dielectric or metallic materials in gas phase reactors. Perfluoro(2-methyl-3-pentanone) is a commonly used fire-fighting fluid.
The photolysis of Perfluoro(2-methyl-3-pentanone) gives CF3CF2C·(O) and ·CF(CF3)2 radicals. This compound can undergo hydrolysis to produce PFPrA and CF3CFHCF3 (HFC-227ea) in a manner analogous to the Haloform reaction. The atmospheric fate of Perfluoro(2-methyl-3-pentanone) seems to be direct photolysis, which, under low NOx conditions, gives PFPrA a small yield[1].
|
|
|
|


