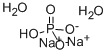|
|
|
| Product Name |
Disodium hydrogen phosphate dihydrate |
| Model No |
SIL042C |
| Cas No. |
10028-24-7 |
| Assay |
99% |
| Molecular Structural |
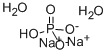 |
| Details: |
Product Name:Disodium hydrogen phosphate dihydrate
CAS:10028-24-7
MF:2Na.HO4P.2H2O
MW:177.99
Melting point :92,5°C
density :1.064 g/mL at 20 °C
vapor density :4.9 (vs air)
solubility :H2O: 0.5 M at 20 °C, clear, colorless
PH:8.9-9.2 (25℃, 0.5M in H2O)
Chemical Properties:The USP 32 states that dibasic sodium phosphate is dried or contains, 1, 2, 7, or 12 molecules of water of hydration. Anhydrous dibasic sodium phosphate occurs as a white powder. The dihydrate occurs as white or almost white, odorless crystals.
The heptahydrate occurs as colorless crystals or as a white granular or caked salt that effloresces in warm, dry air. The dodecahydrate occurs as strongly efflorescent, colorless or transparent crystals.
Uses:Sodium phosphate dibasic dihydrate has been used for isolating DNA and RNA from bacterial samples of human gastrointestinal (GI) tract.
Dibasic sodium phosphate is used in a wide variety of pharmaceutical formulations as a buffering agent and as a sequestering agent. Therapeutically, dibasic sodium phosphate is used as a mild laxative and in the treatment of hypophosphatemia.Dibasic sodium phosphate is also used in food products; for example as an emulsifier in processed cheese. Sodium phosphate dibasic dehydrate is an important component of running buffer of denaturing gel electrophoresis.
Storage:The anhydrous form of dibasic sodium phosphate is hygroscopic. When heated to 40℃, the dodecahydrate fuses; at 100℃ it loses its water of crystallization; and at a dull-red heat (about 240℃) it is converted into the pyrophosphate, Na4P2O7. Aqueous solutions of dibasic sodium phosphate are stable and may be sterilized by autoclaving.
The bulk material should be stored in an airtight container, in a cool, dry place. |
|
|
|